Say hello to MassDevice +5, a bite-sized view of the top five medtech stories of the day. This feature of MassDevice.com’s coverage highlights our 5 biggest and most influential stories from the day’s news to make sure you’re up to date on the headlines that continue to shape the medical device industry.
Get this in your inbox everyday by subscribing to our newsletters.
5. FDA releases May 2016 510(k) clearances
The Food & Drug Administration released the 510(k) clearances it issued in May 2016. Read more
4. Google’s Verily Star Trek “Tricorder” moonshot flops
Verily’s moonshot project which aimed to create a futuristic every-aspect diagnostic device and cancer treatment, much like Star Trek’s “Tricorder” devices, has flopped after 3 years in development. Read more
3. Medtronic teases new “extravascular” ICD system
Medtronic today released results from feasibility studies of its new “extravascular” EV-ICD System implantable cardioverter defibrillator designed with leads placed outside the hearts and veins, under the rib cage. Read more
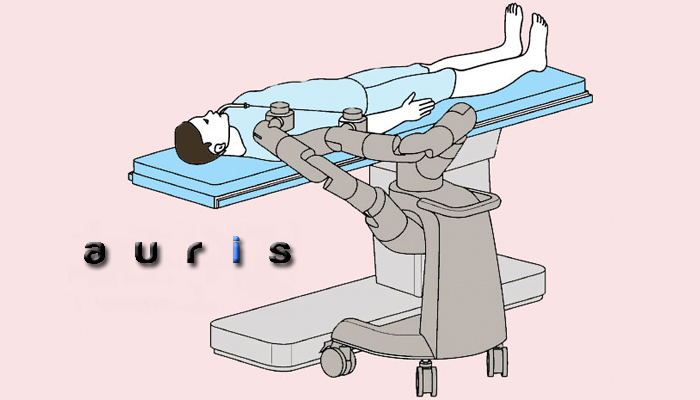
2. FDA clears Ares robot-assisted bronchoscopy device from Auris Surgical Robotics
The FDA last month granted 510(k) clearance to the Ares robot-assisted bronchoscopy platform developed by stealthy Auris Surgical Robotics. Read more
1. Medtronic enters bundled ortho implant space with Responsive Orthopedics buy
Medtronic (NYSE:MDT) said this week that it acquired Responsive Orthopedics for an undisclosed amount, in preparation for its entry into the bundled hip-and-knee market. Read more
The post MassDevice.com +5 | The top 5 medtech stories for June 8, 2016 appeared first on MassDevice.